FDA Briefing Document - Pediatric Oncology Subcommittee of the Oncologic Drugs Advisory Committee (ODAC) June 17 and 18, 2020

SIDP on Twitter: "This just in: FDA advisory committee recommends approval of omadacycline for treatment of ABSSI & CABP. Briefing document here: https://t.co/5y9d8qLUdN https://t.co/aT7Weeu0rI" / Twitter
FDA Briefing Document Psychopharmacologic Drugs Advisory Committee (PDAC) and Drug Safety and Risk Management (DSaRM) Advisory
CT-P10 A PROPOSED BIOSIMILAR TO RITUXAN® FDA ADVISORY COMMITTEE MEETING BRIEFING DOCUMENT ONCOLOGIC DRUGS ADVISORY COMMITTEE Me
FDA Briefing Document - Spark Therapeutics, Inc, LUXTURNATM: Cellular, Tissue, and Gene Therapies Advisory Committee Meeting - O
Availability of Information Given to Advisory Committee Members in Connection with CDRH Open Public Panel Meetings; Draft Guidan
March 28-29, 2023 Joint Meeting of the Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs
![PDF] Ziprasidone Mesylate for Intramuscular Injection 1 Advisory Committee Briefing Document Appendix 1, The Behavioural Activity Rating Scale | Semantic Scholar PDF] Ziprasidone Mesylate for Intramuscular Injection 1 Advisory Committee Briefing Document Appendix 1, The Behavioural Activity Rating Scale | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/608dde6ea19d8f7b35c9876408fad41316408421/6-Table2-1.png)
PDF] Ziprasidone Mesylate for Intramuscular Injection 1 Advisory Committee Briefing Document Appendix 1, The Behavioural Activity Rating Scale | Semantic Scholar
FDA Briefing Document Joint Meeting of Anesthetic and Analgesic Drug Products Advisory Committee and Drug Safety and Risk Manage
Oncologic Drugs Advisory Committee (ODAC) Meeting July 14, 2020 BLA# 761158 Drug Name: Belantamab Mafodotin Applicant: GlaxoSmit

Fillable Online FDA Briefing Document Oncologic Drugs Advisory Committee ... Fax Email Print - pdfFiller
Amylyx Pharmaceuticals Announces Posting of Briefing Documents for FDA Advisory Committee Meeting on AMX0035
FDA Briefing Information for the August 13, 2020 Meeting of the Oncologic Drugs Advisory Committee (AM Session)
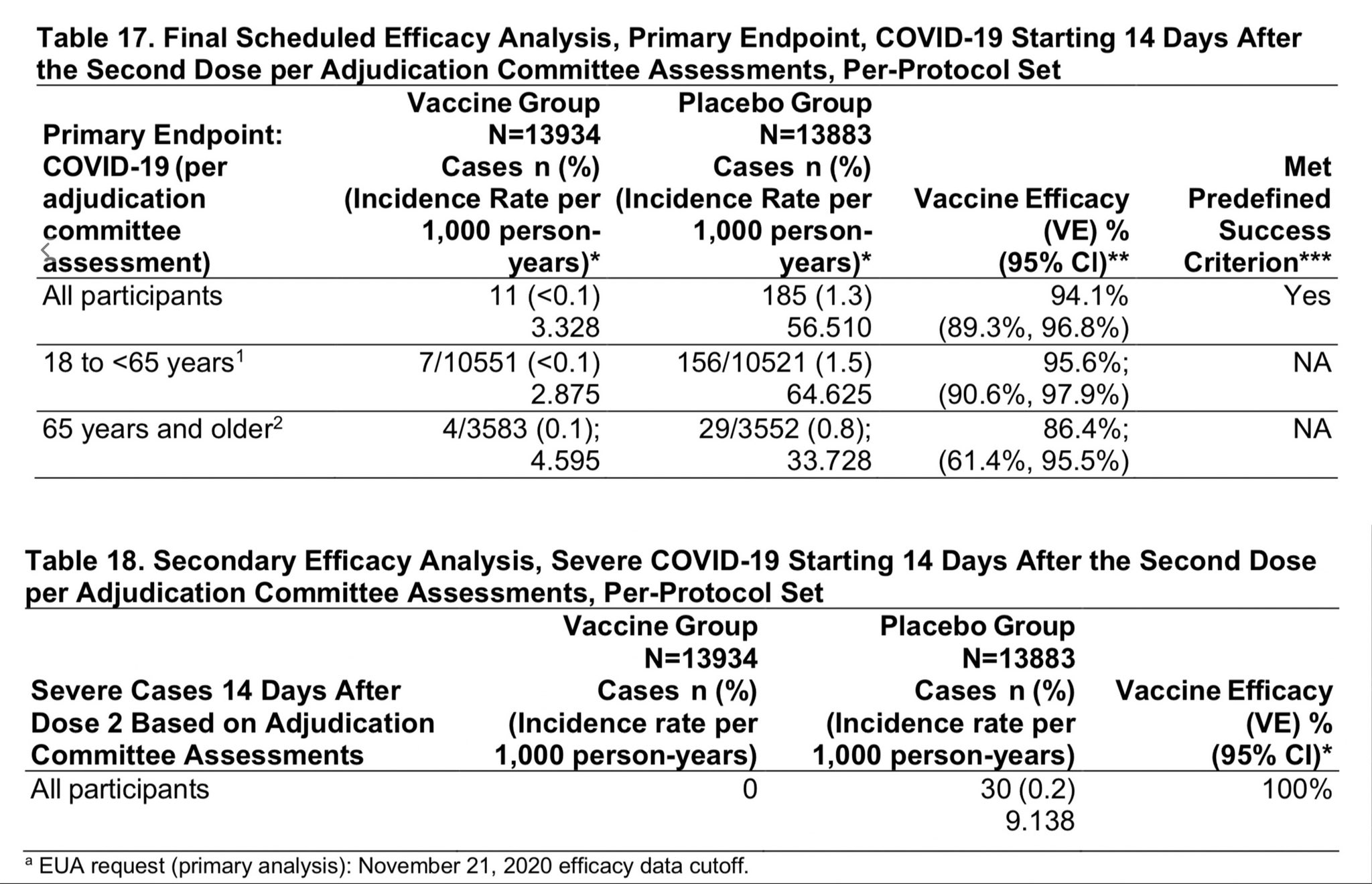
David R. Liu on Twitter: "Want to review #SARSCoV2 #COVID19 vaccine safety and efficacy data yourself? Linked below is the briefing document for the FDA vaccine advisory committee on Moderna's mRNA vaccine,