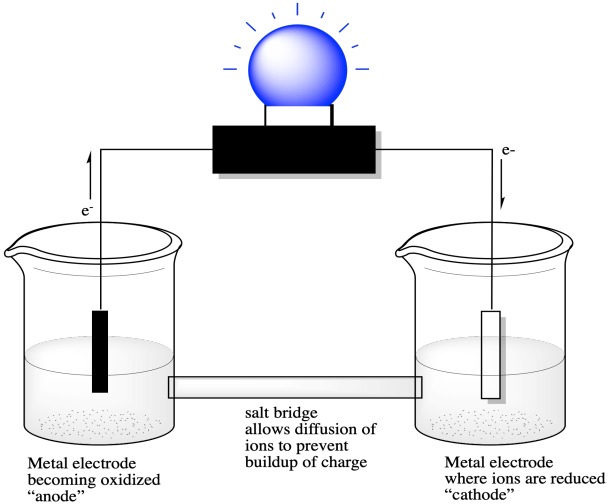
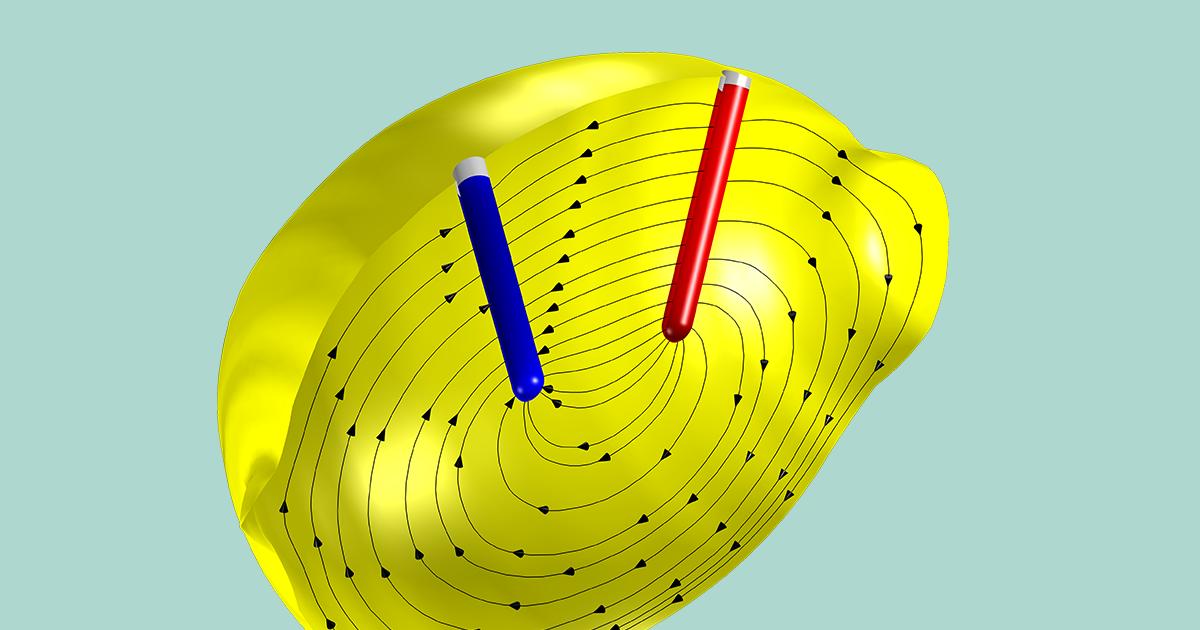
SOLVED: Lemon Battery The balanced oxidation-reduction (redox) reaction for the lemon battery is: Zn (s) + 2 H+ (aq) â†' Zn2+ (aq) + H2 (g). Draw a diagram for the lemon battery

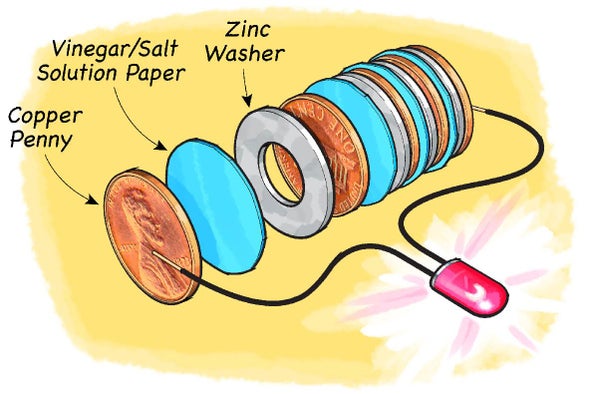
SOLVED: Building Battery Background: Electricity is generated by ions such as H+ in motion. As we have seen in previous chapters, it is produced by acids such as citric acid. Build the

electrochemistry - Why electrons are attracted by cathode in Voltaic/Galvanic cell - Chemistry Stack Exchange

electrochemistry - Why do electrons follow from Zn to Cu but not Cu to Zn in the lemon battery experiment? - Chemistry Stack Exchange



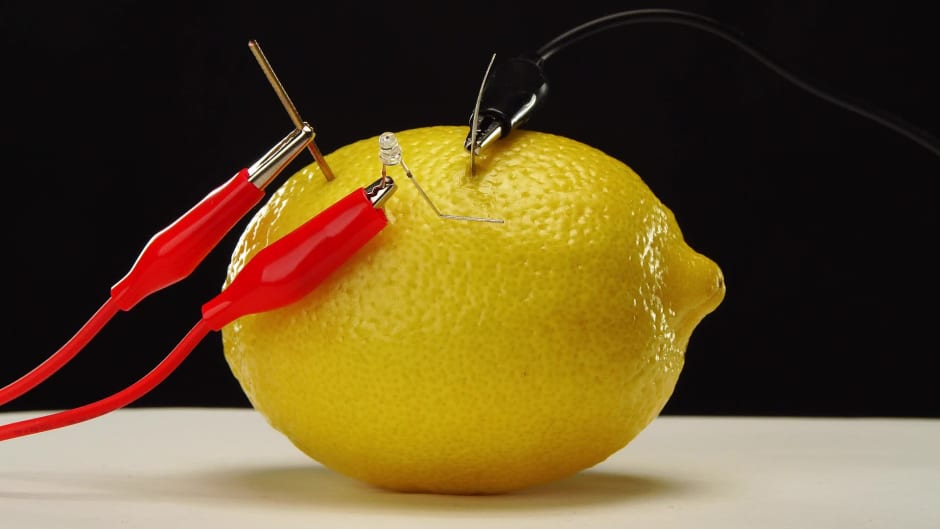



:max_bytes(150000):strip_icc()/lemon-battery-is-a-device-used-in-experiments-proposed-in-many-science-textbooks-around-the-world-it-is-made-by-inserting-two-different-metallic-objects-for-example-galvanized-nail-and-copper-coin-into-lemon-the-copper-coin-serves-as-the-positive-57d2abba3df78c71b637d820.jpg)